

Ice Melting: Once the ice has melted, the water that has been created can be frozen to restore the ice.Most of the chemical changes have irreversible changes.Įxamples of Reversible and Irreversible Change Most of the physical changes have reversible changes. The amount of work done in an irreversible process is always less than the amount of work done in a reversible process.

The amount of work done in a reversible process is more than in an irreversible process. The chemical properties of a substance undergoing an irreversible change are altered. The chemical properties of a substance undergoing a reversible change are not altered. Processes that are irreversible can only go in one direction. Reversible procedures can be carried out in either a forward or backward manner. There is no balance between the system and its surroundings here. Irreversible change is formed by a rather quick procedure. Reversible Change is formed through a long process that goes through several minor stages to keep the system and its environment in balance. Irreversible Changes cannot be reversed and returned to their original state. Reversible Changes can be reversed and returned to their original state. We claim that change cannot be reversed or that the changes are irreversible if the new material generated does not undergo reverse change to form the old substance.ĭifference between Reversible and Irreversible Change When certain agents, like heat, light, electricity, force, and so on, are given to a substance, it transforms into a new substance. They have already been transformed into something new with entirely distinct features.Īnother important point to note is that the individual features are lost in an irreversible process. Reactants can transform into products, but products cannot transform back into reactants. You can no longer acquire the components in their original form, no matter how hard you try.ĭough baked to form bread is an example of an irreversible changeĪnother good example is fuel use, which cannot be reversed after it has been transformed into energy.Ĭhemical processes that are irreversible can only go one way. You start by gathering all of the essential items, such as veggies, spices, and meat, before cooking everything and preparing a dish. These novel materials can be advantageous at times.Ĭonsider the boiling of food as an example of a reversible process to grasp this concept.
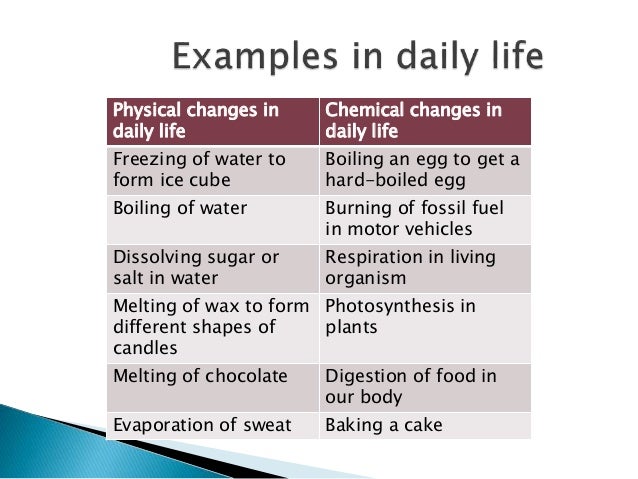
New materials are always produced during an irreversible transition. If a change cannot be reversed, it is said to as irreversible. Water is originally in a liquid condition, then freezes to produce ice, which then melts to return to the water when heated. Let us use the example of ice melting and vice versa to illustrate our point. These factors can cause changes in a substance's shape, size, location, color, or internal structure.Ī reversible process, as the name implies, is one that may be returned to its beginning condition once it has been finished. Reactants can transform into products, and products can transform back into reactants. So many changes are taking place all around us, all the time.Ĭhemical reactions that are reversible can happen in both directions. Some typical changes are the shift of day and night, weather changes, paper burning, changes in children, and changes in flora (germination of seeds, growth, flowering, fruiting, etc.). The majority of the objects in our environment are always changing. The interaction of sodium with water to generate sodium hydroxide and hydrogen is an example of a chemical transition. Some changes are slow or gradual in nature, while others are fast-occurring some are natural in the atmosphere while others are artificial or man-made.Ĭhemical changes occur when a material is combined with another to produce a new substance as a result, a process which we refer to as chemical synthesis, or when a molecule decomposes to result in two or more distinct chemicals, a process known as chemical decomposition. In some circumstances, the original material can be restored, while in others, you can not. In our daily life, we observe a lot of changes such as the melting of the ice, cooking of food, the growth of plants, the dying of leaves, and so on. Changes refer to a random or unpredictable phenomenon that may either be good or bad.
/simple-experiment-58b5b3325f9b586046bbfa7f.jpg)
Change can be explained on physical grounds in chemistry.


 0 kommentar(er)
0 kommentar(er)
